A Low Trophic Position of Japanese Eel Larvae Indicates Feeding on Marine Snow
Nov. 7, 2012
Michael J. Miller1
Yoshito Chikaraishi2
Nanako O. Ogawa2
Yoshiaki Yamada3
Katsumi Tsukamoto1
Naohiko Ohkouchi2
1Atmosphere and Ocean Research Institute, The University of Tokyo
2Institute of Biogeosciences, Japan Agency for Marine-Earth Science and Technology
3IRAGO Institute Co. Ltd.

What eel larvae feed on in the surface layer of the ocean has remained mysterious. Gut contents and bulk nitrogen stable isotope studies suggested that these larvae, called leptocephali, feed at a low level in the oceanic food web, whereas other types of evidence have suggested small zooplankton are eaten. In this study we determined the nitrogen isotopic composition of amino acids of both natural larvae and laboratory reared larvae of the Japanese eel to estimate the trophic position (TP) of leptocephali. We observed a mean TP of 2.4 for natural leptocephali, which is consistent with feeding on particulate organic matter (POM) such as marine snow and discarded appendicularian houses containing bacteria, protozoans and other biological materials. The nitrogen isotope enrichment values of the reared larvae confirm that the primary food source of natural larvae is only consistent with POM. This shows that leptocephali feed on readily available particulate material originating from various sources closely linked to ocean primary production and that leptocephali are a previously unrecognized part of oceanic POM cycling.
RESEARCH PAPER SUMMARY![]() (358KB)
(358KB)
BACKGROUND
Leptocephali are the larvae of anguillid eels (Figure 1), and marine eels and their close relatives, which are a phylogenetically distinct group of fishes (Inoue et al., 2011). The bodies of leptocephali are highly transparent and they grow to large sizes, but historically no food could be seen in their thin guts. This led to the hypothesis that maybe these larvae did not feed through the mouth, but absorbed dissolved organic nutrients through their thin skin instead. Eventually materials that were determined to be components of particulate organic matter (POM) or marine snow, which is widely present in the ocean (Alldredge and Silver, 1988), were microscopically observed in the guts of eel larvae (Otake et al., 1993, Mochioka and Iwamizu, 1996) and were more clearly photographed recently (Miller et al., 2012a). Bulk stable Nitrogen isotope studies on leptocephali suggested they feed on low trophic level materials within the marine food web (Otake et al., 1993; Miyazaki et al., 2011), but the isotopic fractionation values were not known for this type of larvae. In addition, ciliates were observed in their guts (Govoni, 2010), and a recent DNA barcoding study found a variety of DNA sequences of marine organisms in the guts of small European eel leptocephali, Anguilla angulla, collected in the Sargasso Sea including those of gelatinous zooplankton (Riemann et al., 2010), many of which are predators on other zooplankton. In addition, DNA sequences of fungi were found recently in the guts of Japanese eel leptocephali (Terahara et al., 2011). These various types of information made it unclear how leptocephali obtain their nutrition in the ocean environments where they live worldwide.
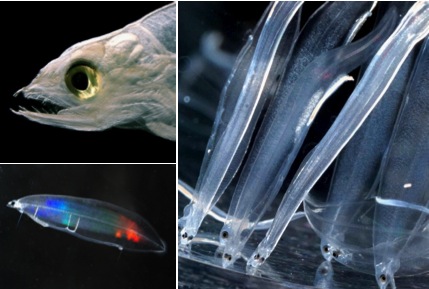
Figure. 1. Photographs of leptocephali of freshwater eels of the genus Anguilla, showing the head of a giant mottled eel larva, Anguilla marmorata, from the western North Pacific (upper left) and artificially cultured Japanese eel larvae, Anguilla japonica, in the laboratory (bottom and right, courtesy of Y. Yamada, IRAGO Institute), which are like those used in this study to validate the trophic difference between the larvae and their food source. Red and blue colors are light-reflections in the bottom left photo.
FINDINGS OF THE STUDY
Our study used the new technique of amino acid δ15N isotope analysis (McClelland and Montoya, 2002; Chikaraishi et al., 2007, 2009; and other studies) to solve the problem of what leptocephali feed on. We used both natural larvae caught near their spawning area and two groups of artificially spawned and reared larvae of the Japanese eel, which is a freshwater eel in East Asia that spawns offshore in the western North Pacific (Tsukamoto et al., 2011), and its larvae have been reared in the laboratory recently (Tanaka et al., 2001). Our objective was to estimate the trophic position of natural leptocephali within the oceanic food web using amino acid isotopes of natural and cultured larvae to validate the food source of natural leptocephali.
Natural and laboratory reared leptocephali were analyzed for their nitrogen isotopic composition of phenylalanine (δ15NPhe) and glutamic acid (δ15NGlu) to estimate their trophic position, because these two amino acid have been found to be the most useful for this analysis. The two diets of the artificially reared larvae were also analyzed, but their diets include shark egg yolks from high trophic levels (Figure 2). The trophic position of each specimen was calculated using the established formula (Chikaraishi et al., 2009): Trophic Position = (δ15NGlu – δ15NPhe – 3.4)/7.6 + 1. For comparison, specimens of other marine organisms were also analyzed, and some values from the literature were included (Figure 3).
These analyses in relation to the other organisms that were also examined clearly showed that the trophic position of natural leptocephali was very low in the marine food web, at a level of 2.4 (Figure 2, 3). This indicated that the food of leptocephali must be between levels 1 and 2, which would be between primary producers and primary consumers. This level is only consistent with leptocephali feeding on marine snow.
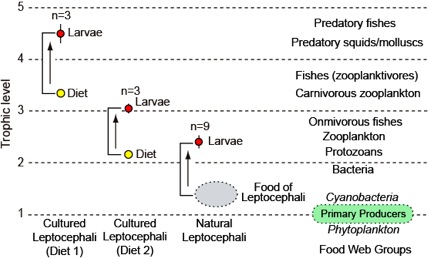
Figure. 2. The trophic positions of natural Japanese eel leptocephali, and cultured leptocephali and their artificial diets, in relation to the approximate trophic levels of other general types of marine organisms in the oceanic food web. Modified from Miller et al. (2012b, Biology Letters).
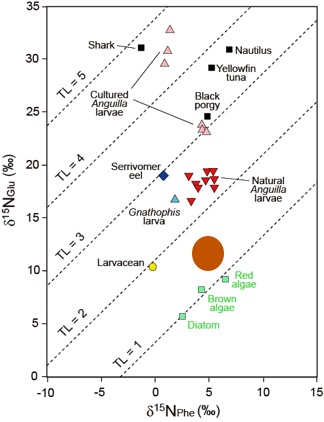
Figure 3. Plot of the nitrogen isotopic compositions of phenylalanine (δ15NPhe) and glutamic acid (δ15NGlu) of the specimens of this study and the estimated trophic position of the food of natural leptocephali (brown circle) in relation to the trophic levels (diagonal lines) that are determined by the δ15NPhe and δ15NGlu values. TL = trophic level. Modified from Miller et al. (2012b, Biology Letters).
SIGNIFICANCE OF THE STUDY
The trophic position of 2.4 that we determined for natural Japanese eel indicates that leptocephali feed on particulate organic matter such as marine snow. Marine snow consists of the frequently discarded larvacean (appendicularian) houses, bacteria, protozoans, other colonizing small organisms, carbohydrates and other biological materials (Figure 4; Alldredge and Silver, 1988). Production of marine snow is closely linked to primary production in the ocean, so feeding on this type of material has a variety of important implications because most fish larvae feed on zooplankton.
The validation that marine snow is the primary food of leptocephali is especially significant because the Japanese eel is an important food species in Japan and other parts of East Asia, and there are intensive efforts to raise the larvae in the laboratory to establish aquaculture procedures for leptocephali to produce seedling glass eels for aquaculture. If successful this would take pressure off the wild eel populations whose glass eels are captured to use in aquaculture. Efforts are also underway to rear eel larvae of other species now in Europe, the US, S. Korea, and New Zealand. Knowing what the natural food of leptocephali is may be able to aid efforts to find a successful food to feed these larvae that are presently being fed with food containing shark egg yolks; which somehow attracts the larvae to eat it, but is not a natural food with good performance for growth and survival. These efforts have succeeded to produce glass eels, but still not at the industrial scale that is urgently needed when the Japanese eel population is drastically declining recently.
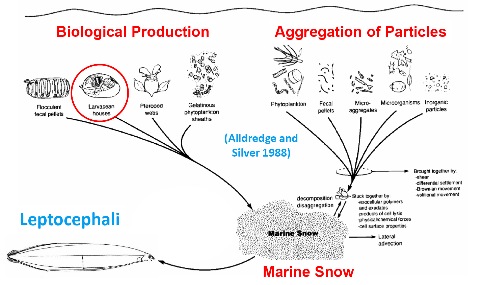
Figure 4. An overview of the sources of material that aggregates into marine snow modified from Alldredge and Silver (1988). One component consists of discarded larvacean (appendicularian) houses (red circle), which are frequently abandoned when their filters clog, and these houses may be specifically targeted as food by leptocephali along with marine snow in general.
There is also great interest in anguillid eels in Europe and the rest of the world now because of the drastic declines worldwide. One possible aspect of the declines is that changes in productivity in the ocean may have been caused by climatic changes or ocean-atmosphere regime shifts (Miller et al., 2009), which could affect the composition or availability of marine snow. Understanding the diet of these unusual larvae may be important to know what is causing the global declines in anguillid eels.
Leptocephali of the almost 800 species of marine and freshwater eels worldwide appear to be more widely present and abundant than generally realized, so a new awareness that these larvae are eating marine snow has implications for better understanding particulate matter cycling in the ocean and the global ocean carbon cycle, because all leptocephali eventually recruit to other environments ranging from freshwater to the deep-sea (Miller, 2009). Future research will provide more information about the feeding ecology of these remarkable larvae to better understand their role in marine ecosystems.
REFERENCES
Alldredge, A. L and Silver, M. W. 1988. Characteristics, dynamics and significance of marine snow. Progr. Oceanogr. 20: 41–82.
Chikaraishi, Y., Kashiyama, Y., Ogawa, N. O., Kitazato, H. and Ohkouchi, N. 2007. Metabolic control of nitrogen isotope composition of amino acids in macroalgae and gastropods: implications for aquatic food web studies. Mar. Ecol. Prog. Ser. 342: 85–90.
Chikaraishi, Y., Ogawa, N.O., Kashiyama, Y., Takano, Y., Suga, H., Tomitani, A., Miyashita, H. Kitazato, H. and Ohkouchi, N. 2009. Determination of aquatic food-web structure based on compound-specific nitrogen isotopic composition of amino acids. Limnol. Oceanogr. Meth. 7: 740–750.
Govoni, J. 2010. Feeding on protists and particulates by the leptocephali of the worm eels Myrophis spp. (Teleostei, Anguilliformes, Ophichthidae), and the potential energy contribution of large aloricate protozoa. Scientia Marina 74: 339–344.
Inoue J. G., Miya, M., Miller, M. J., Sado, T., Hanel, R., López, J. A., Hatooka, K., Aoyama, J., Minegishi, Y., Nishida, M., and Tsukamoto, K.. 2010. Deep-ocean origin of the freshwater eels. Biol. Lett. 6: 363–366.
McClelland, J. W. and Montoya, J. P. 2002. Trophic relationships and the nitrogen isotopic composition of amino acids in plankton. Ecology 83: 2173–2180.
Miller, M. J. 2009. Ecology of anguilliform leptocephali: remarkable transparent fish larvae of the ocean surface layer. Aqua-BioSci. Monogr. 2: 1–94.
Miller, M. J., Kimura, S., Friedland, K. D., Knights, B., Kim, H., Jellyman, D. J. and Tsukamoto, K. 2009. Review of ocean-atmospheric factors in the Atlantic and Pacific oceans influencing spawning and recruitment of anguillid eels. Pages 231–249 In: Haro, A. et al. editors. Challenges for Diadromous Fishes in a Dynamic Global Environment. Amer. Fisheries Soc., Symp. 69, Bethesda Maryland.
Miller, M. J., Otake, T., Aoyama, J., Wouthuyzen, S., Suharti, S., Sugeha, H. Y. and Tsukamoto, K. 2012a. Observations of gut contents of leptocephali in the North Equatorial Current and Tomini Bay, Indonesia. Coast. Mar. Sci. 35: 277–288.
Miller, M. J., Chikaraishi Y., Ogawa N. O., Yamada Y., Tsukamoto K. and Ohkouchi N. 2012b. A low trophic position of Japanese eel larvae indicates feeding on marine snow. Biol. Lett. doi: 10.1098/rsbl.2012.0826, published online 7 November.
Miyazaki, S., Kim, H.–Y., Zenimoto, K., Kitagawa, T., Miller, M. J. and Kimura, S. 2011. Stable isotope analysis of two species of anguilliform leptocephali (Anguilla japonica and Ariosoma major) relative to their feeding depth in the North Equatorial Current region. Mar. Biol. 158: 2555–2564.
Mochioka, N. and Iwamizu, M. 1996. Diet of anguillid larvae: leptocephali feed selectively on larvacean houses and fecal pellets. Mar. Biol. 125: 447–452.
Otake, T., Nogami, K. and Maruyama, K. 1993. Dissolved and particulate organic matter as possible food sources for eel leptocephali. Mar. Ecol. Progr. Ser. 92: 27–34.
Riemann, L., Alfredsson, H., Hansen, M. M., Als, T. D., Nielsen, T. G., Munk, P., Aarestrup, K., Maes, G. E., Sparholt, H., Petersen, M. I., Bachler, M. and Castonguay, M. 2010. Qualitative assessment of the diet of European eel larvae in the Sargasso Sea resolved by DNA barcoding. Biol. Lett. 6: 819–822.
Tanaka, H., Kagawa, H. and Ohta, H. 2001. Production of leptocephali of Japanese eel (Anguilla japonica) in captivity. Aquaculture 201: 51–60.
Terahara, T., Chow, S., Kurogi, H., Lee, S.-H., Tsukamoto, K., Mochioka, N., Tanaka, H. and Takeyama, H. 2011. Efficiency of peptide nucleic acid-directed PCR clamping and its application in the investigation of natural diets of the Japanese eel leptocephali. PLoS ONE 6(11): e25715.doi:10.1371/journal.pone.0025715.
Tsukamoto, K., Chow, S., Otake, T., Kurogi, H., Mochioka, N., Miller, M.J., Aoyama, J., Kimura, S., et al. 2011. Oceanic spawning ecology of freshwater eels in the western North Pacific. Nature Commun. 2, 179. doi: 10.1038/ncomms1174.
![]()



